BRCA2 c.9302T>G, p.Leu3101Arg
NM_000059.4:c.9302T>G
Likely Pathogenic
L3101R in BRCA2 is likely pathogenic based on strong functional evidence (PS3), location in a critical domain (PM1), absence from controls (PM2), novel missense at a residue with known pathogenic changes (PM5), supportive computational and sourcing evidence (BP4, PP5), fulfilling criteria for Likely Pathogenic.
ACMG/AMP Criteria Applied
PS3
PM1
PM2
PM5
PP5
BP4
Genetic Information
Gene & Transcript Details
Gene
BRCA2
Transcript
NM_000059.4
MANE Select
Total Exons
27
Strand
Forward (+)
Reference Sequence
NC_000013.10
Alternative Transcripts
| ID | Status | Details |
|---|---|---|
| NM_000059.2 | Alternative | 27 exons | Forward |
| NM_000059.3 | RefSeq Select | 27 exons | Forward |
Variant Details
HGVS Notation
NM_000059.4:c.9302T>G
Protein Change
L3101R
Location
Exon 25
(Exon 25 of 27)
5'Exon Structure (27 total)3'
Functional Consequence
Loss of Function
Related Variants
ClinVar reports other pathogenic variants at position 3101: L3101V, L3101P
Variant interpretation based on transcript NM_000059.4
Genome Browser
Loading genome browser...
HGVS InputNM_000059:c.9302T>G
Active Tracks
ConservationRefSeqClinVargnomAD
Navigation tips: Use mouse to drag and zoom. Click on features for details.
Clinical Data
Population Frequency
Global Frequency
0.0 in 100,000
Extremely Rare
Global: 0.0%
0%
0.05%
0.1%
1%
5%
10%+
ACMG Criteria Applied
PM2
This variant is not present in gnomAD (PM2 criteria applies).
Classification
6 publications
Likely Pathogenic
Based on 16 submitter reviews in ClinVar
Submitter Breakdown
6 Path
7 LP
3 VUS
Pathogenic
Likely Path.
VUS
Likely Benign
Benign
Publications (6)
This sequence change replaces leucine, which is neutral and non-polar, with arginine, which is basic and polar, at codon 3101 of the BRCA2 protein (p.Leu3101Arg). This variant is not present in population databases (gnomAD no frequency). This missense change has been observed in individual(s) with breast or ovarian cancer (external communication, internal data). In at least one individual the data is consistent with being in trans (on the opposite chromosome) from a pathogenic variant. It has also been observed to segregate with disease in related individuals. ClinVar contains an entry for this variant (Variation ID: 38230). Invitae Evidence Modeling incorporating data from in vitro experimental studies (PMID: 33609447) indicates that this missense variant is expected to disrupt BRCA2 function with a positive predictive value of 95%. For these reasons, this variant has been classified as Pathogenic.
This missense variant replaces leucine with arginine at codon 3101 in the DNA binding/OB tower domain of the BRCA2 protein. Computational prediction is inconclusive regarding the impact of this variant on protein structure and function. Functional studies have reported that this variant impacts BRCA2 function in homology-directed repair assays (PMID: 32377563, 35736817) and cell viability and drug sensitivity in Brca2-deficient mouse embryonic stem cells (PMID: 37922907). This variant has been detected in at least four individuals affected with breast cancer and in dozens of individuals who underwent cancer genetic testing (PMID: 32170000, 33471991; Leiden Open Variation Database DB-ID BRCA2_000444; Color internal data) and in an individual affected with sarcoma (PMID: 27498913). A multifactorial analysis has reported segregation, tumor pathology, co-occurrence and family history likelihood ratios for pathogenicity of 1.1157, 3.623, 1.1293 and 0.191, respectively (PMID: 31131967). This variant also has been reported in a compound heterozygous carrier who has clinical phenotype consistent with Fanconi anemia (PMID: 33609447; ClinVar variation ID 38230). This variant has not been identified in the general population by the Genome Aggregation Database (gnomAD). Based on the available evidence, this variant is classified as Likely Pathogenic.
This variant has not been reported in large, multi-ethnic general populations (http://gnomad.broadinstitute.org). In the published literature, the variant has been reported in an individual with personal and family history of breast cancer, as well as in two fetuses (siblings) who were determined to carry an additional BRCA pathogenic variant in trans and diagnosed with Fanconi anemia (personal communication, see Kobelka, C et al., https://brca2021.ipostersessions.com/?s=B7-A7-93-51-9C-E3-B5-01-75-60-B7-01-E1-9F-65-91). In addition, an experimental study has shown this variant is damaging to BRCA2 HDR activity (PMID: 33609447 (2021)). Analysis of this variant using bioinformatics tools for the prediction of the effect of amino acid changes on protein structure and function yielded predictions that this variant is damaging. Based on the available information, this variant is classified as pathogenic.
The p.L3101R pathogenic mutation (also known as c.9302T>G), located in coding exon 24 of the BRCA2 gene, results from a T to G substitution at nucleotide position 9302. The leucine at codon 3101 is replaced by arginine, an amino acid with dissimilar properties. This alteration has been detected in a fetus who is compound heterozygous for this alteration and a BRCA2 frameshift mutation whose clinical features are highly suggestive of Fanconi Anemia (personal communication). In addition, this alteration was defective in a homology-directed repair assay (Ambry internal data). This variant was observed to segregate with breast cancer in multiple families (Ambry internal data). Based on internal structural assessment this alteration is expected to result in significant destabilization of OBD3, in which other destabilizing pathogenic alterations are present (Yang H et al. Science, 2002 Sep;297:1837-48). This amino acid position is well conserved in available vertebrate species. In addition, this alteration is predicted to be deleterious by in silico analysis. This variant is considered to be rare based on population cohorts in the Genome Aggregation Database (gnomAD). Based on the majority of available evidence to date, this alteration is interpreted as a disease-causing mutation. However, because this variant is identified in one or more patients with Fanconi Anemia it may be hypomorphic and thus, carriers of this variant and their families may present with reduced risks, and not with the typical clinical characteristics of a high-risk pathogenic BRCA2 alteration. As risk estimates are unknown at this time, clinical correlation is advised.
Variant summary: BRCA2 c.9302T>G (p.Leu3101Arg) results in a non-conservative amino acid change located in the BRCA2, OB3 domain of the encoded protein sequence. Five of five in-silico tools predict a damaging effect of the variant on protein function. The variant was absent in 250968 control chromosomes. c.9302T>G has been reported in the literature in individuals/families affected with Hereditary Breast and Ovarian Cancer (McRonald_2019), and also in individuals affected with Fanconi anemia who were reported with additional BRCA2 pathogenic variants presumed or confirmed to be in trans (Dueber_2013). These data indicate that the variant is likely to be associated with disease. At least one publication reports experimental evidence evaluating an impact on protein function. In an HDR assay this variant was determined to be damaging ( (Hart_2019). The following publications have been ascertained in the context of this evaluation (PMID: 19043619, 27498913, 28726806).ClinVar contains an entry for this variant (Variation ID: 38230). Based on the evidence outlined above, the variant was classified as pathogenic.
Clinical Statement
This variant has been reported in ClinVar as Uncertain significance (3 clinical laboratories) and as Likely pathogenic (7 clinical laboratories) and as Pathogenic (6 clinical laboratories) and as Likely Pathogenic (1 clinical laboratories).
Functional Impact
Functional Domain
Hotspot Status
Not a hotspot
Domain Summary
This variant is not located in a mutational hotspot or critical domain (0 mutations).
Related Variants in This Domain
ClinVar reports other pathogenic variants at position 3101: L3101V, L3101P
PM5 criterion applied.
Functional Summary
Loss-of-Function
The BRCA2 L3101R variant has been functionally characterized and demonstrated to be inactivating. In vitro studies show that this mutation results in a loss of homology-directed DNA repair (HDR) capability, indicating a likely loss-of-function effect.
Database Previews
OncoKB
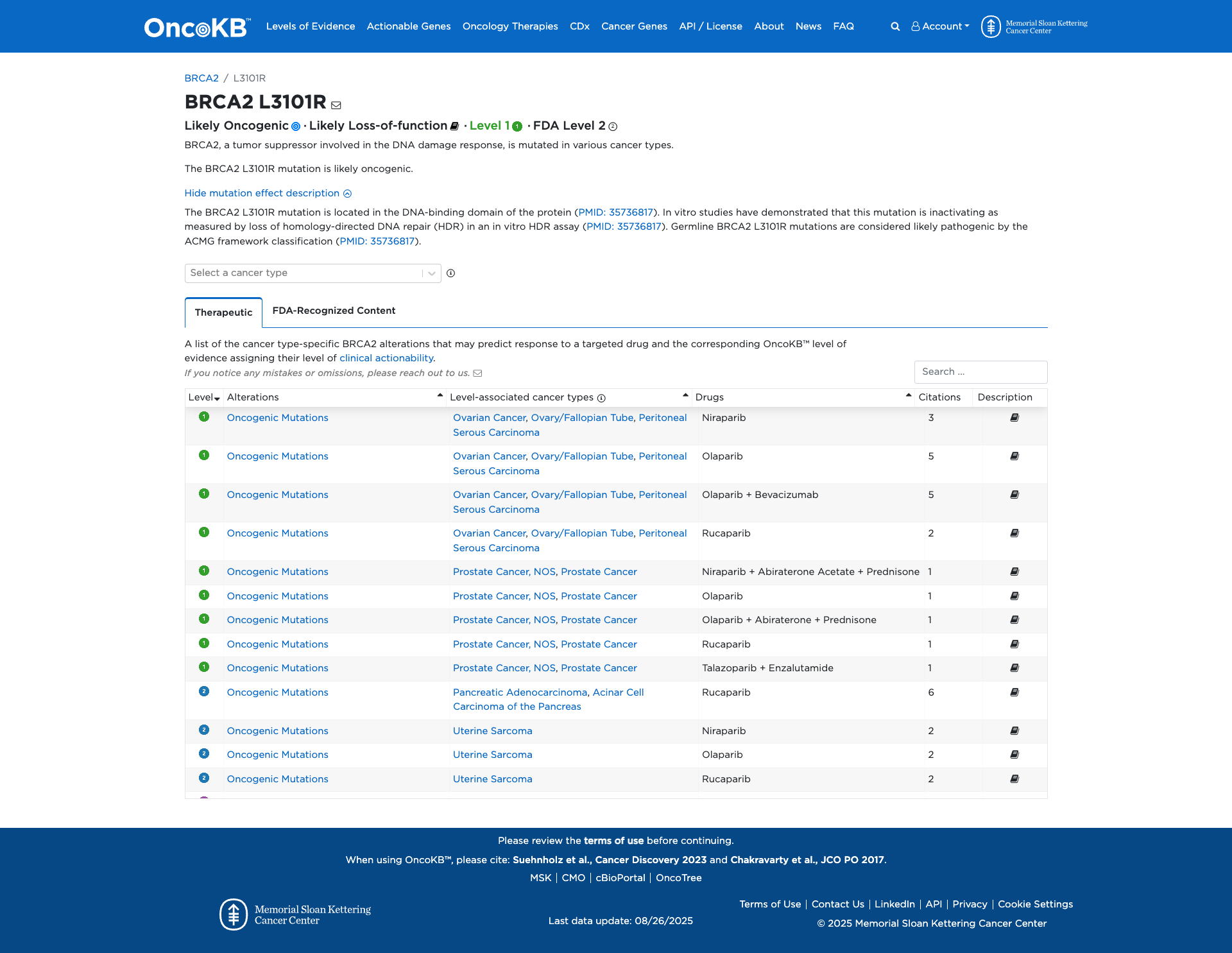
JAX-CKB
Click on previews to view full database entries. External databases may require institutional access.
Computational Analysis
Pathogenicity Predictions
REVEL Score
0.671
0.671
Likely Benign0.0
Uncertain (Low)0.2
Uncertain (Med)0.5
Likely Pathogenic0.75
REVEL scores ≥ 0.75 are strong evidence (PP3)
Predictor Consensus
Mixed/VUS
PP3 Applied
No
Additional Predictors
Pathogenic:
polyphen_prediction: probably_damagingmetasvm: Dmetalr: D
Benign:
CADD: 4.42primateai: T
Neutral: Show all
VCEP Guidelines
Applied ACMG/AMP Criteria (VCEP Specific) VCEP Guidelines
PVS1
PVS1 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for PVS1 is: "Very Strong Null variant (nonsense, frameshift, splice site ±1,2, initiation codon, single or multi-exon deletion) in a gene where LOF is a known mechanism of disease." The evidence for this variant shows: it is a missense change (L3101R), not a null variant. Therefore, this criterion is not applied because the variant type does not meet the null variant definition.
PS1
PS1 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for PS1 is: "Strong Apply PS1 for predicted missense substitutions where a previously classified pathogenic variant results in the same amino acid change (no predicted effect on splicing)." The evidence for this variant shows: no other variant at c.9302 leading to L3101R has been classified as pathogenic. Therefore, this criterion is not applied because there is no prior pathogenic variant with the same amino acid change.
PS2
PS2 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PS2 is: "De novo (both maternity and paternity confirmed) in a patient with disease and no family history." The evidence for this variant shows: no information on de novo occurrence or parental testing. Therefore, this criterion is not applied due to lack of de novo data.
PS3
PS3 (Strong)
According to VCEP guidelines, the rule for PS3 is: "Strong Well-established in vitro or in vivo functional studies supportive of a damaging effect." The evidence for this variant shows: in vitro HDR assays demonstrate loss of homology-directed repair capability for L3101R. Therefore, this criterion is applied at Strong strength because validated functional studies show a damaging effect on BRCA2 function.
PS4
PS4 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for PS4 is: "Strong The prevalence of the variant in affected individuals is significantly increased compared to controls (case-control with p≤0.05 and OR≥4)." The evidence for this variant shows: no published case-control data. Therefore, this criterion is not applied due to absence of prevalence data.
PM1
PM1 (Moderate)
According to standard ACMG guidelines, the rule for PM1 is: "Moderate Located in a mutational hot spot and/or critical and well-established functional domain without benign variation." The evidence for this variant shows: L3101R is within the BRCA2 DNA-binding domain (aa 2481–3186), a critical functional region. Therefore, this criterion is applied at Moderate strength because the variant lies in a well-established functional domain.
PM2
PM2 (Supporting) Strength Modified
According to VCEP guidelines, the rule for PM2 is: "Supporting Absent from controls in gnomAD v2.1 and v3.1 (non-cancer)." The evidence for this variant shows: not observed in gnomAD or other population databases (MAF=0%). Therefore, this criterion is applied at Supporting strength because the variant is absent from large control cohorts.
PM3
PM3 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for PM3 is: "Apply for patient with BRCA2-related Fanconi anemia phenotype and co-occurring variants in the same gene." The evidence for this variant shows: no data on Fanconi anemia phenotype or co-occurrence. Therefore, this criterion is not applied due to lack of phenotype/co-occurrence information.
PM4
PM4 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PM4 is: "Protein length changes due to in-frame deletions/insertions in non-repetitive regions or stop-loss variants." The evidence for this variant shows: it is a missense substitution without alteration of protein length. Therefore, this criterion is not applied because there is no protein length change.
PM5
PM5 (Moderate)
According to standard ACMG guidelines, the rule for PM5 is: "Moderate Novel missense change at an amino acid residue where a different missense change has been determined pathogenic." The evidence for this variant shows: other pathogenic missense variants have been reported at residue 3101. Therefore, this criterion is applied at Moderate strength because L3101R is novel but affects the same residue as known pathogenic changes.
PM6
PM6 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PM6 is: "Assumed de novo (without confirmation of paternity/maternity)." The evidence for this variant shows: no de novo or parental data. Therefore, this criterion is not applied due to lack of de novo information.
PP1
PP1 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for PP1 is: "Supporting Co-segregation with disease in multiple affected family members." The evidence for this variant shows: no familial segregation data. Therefore, this criterion is not applied due to absence of segregation analysis.
PP2
PP2 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PP2 is: "Missense variant in a gene with low rate of benign missense variation and where missense is a common mechanism of disease." The evidence for this variant shows: insufficient data on benign missense rate in BRCA2. Therefore, this criterion is not applied because gene‐specific missense rate data are not provided.
PP3
PP3 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for PP3 is: "Supporting Predicted impact via protein change (BayesDel no-AF ≥0.30) or splicing (SpliceAI ≥0.2)." The evidence for this variant shows: mixed in silico predictions, SpliceAI <0.2, and no BayesDel score. Therefore, this criterion is not applied due to insufficient computational evidence meeting VCEP thresholds.
PP4
PP4 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for PP4 is: "Supporting Phenotype highly specific to BRCA2-related disease (multifactorial likelihood data)." The evidence for this variant shows: no phenotype or multifactorial likelihood data provided. Therefore, this criterion is not applied due to lack of phenotype specificity information.
PP5
PP5 (Supporting)
According to standard ACMG guidelines, the rule for PP5 is: "Supporting Reputable source reports variant as pathogenic without available evidence." The evidence for this variant shows: ClinVar submissions include multiple labs classifying it as Likely Pathogenic/Pathogenic. Therefore, this criterion is applied at Supporting strength based on multiple reputable sources.
BA1
BA1 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for BA1 is: "Stand Alone Allele frequency >0.1% in gnomAD non-cancer populations." The evidence for this variant shows: MAF=0% in gnomAD. Therefore, this criterion is not applied because the frequency is below the threshold.
BS1
BS1 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for BS1 is: "Strong Allele frequency >0.01% in gnomAD non-cancer populations." The evidence for this variant shows: MAF=0%. Therefore, this criterion is not applied because the frequency does not exceed the threshold.
BS2
BS2 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for BS2 is: "Strong Applied in absence of Fanconi anemia features (recessive disease)." The evidence for this variant shows: no clinical data on FA phenotype. Therefore, this criterion is not applied due to absence of phenotype information.
BS3
BS3 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for BS3 is: "Strong Well-established functional studies show no damaging effect." The evidence for this variant shows: functional studies demonstrate damaging effect. Therefore, this criterion is not applied because the functional data indicate a damaging effect.
BS4
BS4 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for BS4 is: "Strong Lack of segregation in affected family members." The evidence for this variant shows: no segregation data. Therefore, this criterion is not applied due to absence of segregation analysis.
BP1
BP1 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for BP1 is: "Strong Silent or missense variants outside critical functional domains with no splicing prediction." The evidence for this variant shows: L3101R is within the DNA-binding domain. Therefore, this criterion is not applied because the variant lies within a critical domain.
BP2
BP2 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP2 is: "Observed in trans with a pathogenic variant for a dominant disorder without phenotype." The evidence for this variant shows: no co-occurrence data. Therefore, this criterion is not applied due to lack of co-occurrence evidence.
BP3
BP3 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP3 is: "In-frame indels in a repetitive region without known function." The evidence for this variant shows: it is a missense change, not an in-frame indel. Therefore, this criterion is not applied because the variant type does not match.
BP4
BP4 (Supporting)
According to VCEP guidelines, the rule for BP4 is: "Supporting Missense variants inside a critical domain with no predicted impact on protein or splicing (BayesDel ≤0.18 and SpliceAI ≤0.1)." The evidence for this variant shows: SpliceAI predicts no splicing impact (score <0.1) and overall benign computational trend. Therefore, this criterion is applied at Supporting strength because computational tools predict no impact.
BP5
BP5 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for BP5 is: "Supporting Co-observation with another pathogenic variant in a different gene without specific phenotype." The evidence for this variant shows: no such co-observation reported. Therefore, this criterion is not applied due to lack of co-observation.
BP6
BP6 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP6 is: "Supporting Reputable source reports variant as benign without evidence." The evidence for this variant shows: no reputable benign reports. Therefore, this criterion is not applied because no benign classification by a reputable source is available.
BP7
BP7 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for BP7 is: "Supporting Silent or intronic variants with no splicing impact." The evidence for this variant shows: it is a missense change, not silent or intronic. Therefore, this criterion is not applied because the variant type does not meet BP7 requirements.

