TP53 c.853G>A, p.Glu285Lys
NM_000546.5:c.853G>A
COSMIC ID: COSM10722
Pathogenic
This variant is classified as Pathogenic based on strong functional evidence (PS3), moderate evidence from a known pathogenic residue change (PM5), and supporting evidence from rarity in controls (PM2), computational predictions (PP3), and multiple reputable clinical submissions (PP5).
ACMG/AMP Criteria Applied
PS3
PM2
PM5
PP3
PP5
Genetic Information
Gene & Transcript Details
Gene
TP53
Transcript
NM_000546.6
MANE Select
Total Exons
11
Strand
Reverse (−)
Reference Sequence
NC_000017.10
Alternative Transcripts
| ID | Status | Details |
|---|---|---|
| NM_000546.5 | RefSeq Select | 11 exons | Reverse |
| NM_000546.3 | Alternative | 11 exons | Reverse |
| NM_000546.4 | Alternative | 11 exons | Reverse |
| NM_000546.2 | Alternative | 11 exons | Reverse |
Variant Details
HGVS Notation
NM_000546.5:c.853G>A
Protein Change
E285K
Location
Exon 8
(Exon 8 of 11)
5'Exon Structure (11 total)3'
Functional Consequence
Loss of Function
Related Variants
ClinVar reports other pathogenic variants at position 285: E285V
Alternate Identifiers
COSM10722
Variant interpretation based on transcript NM_000546.6
Genome Browser
Loading genome browser...
HGVS InputNM_000546:c.853G>A
Active Tracks
ConservationRefSeqClinVargnomAD
Navigation tips: Use mouse to drag and zoom. Click on features for details.
Clinical Data
Population Frequency
Global Frequency
0.0 in 100,000
Extremely Rare
Global: 0.0%
0%
0.05%
0.1%
1%
5%
10%+
ACMG Criteria Applied
PM2
This variant is not present in gnomAD (PM2 criteria applies).
Classification
2 publications
Likely Pathogenic
Based on 6 submitter reviews in ClinVar
Submitter Breakdown
3 Path
3 LP
Pathogenic
Likely Path.
VUS
Likely Benign
Benign
Publications (2)
This sequence change replaces glutamic acid, which is acidic and polar, with lysine, which is basic and polar, at codon 285 of the TP53 protein (p.Glu285Lys). This variant is not present in population databases (gnomAD no frequency). This missense change has been observed in individual(s) with breast cancer and sarcoma (PMID: 11051239, 22507745, 23894400). ClinVar contains an entry for this variant (Variation ID: 420133). Advanced modeling performed at Invitae incorporating data from internal and/or published experimental studies (PMID: 12826609, 29979965, 30224644) indicates that this missense variant is expected to disrupt TP53 function with a positive predictive value of 97.5%. Experimental studies have shown that this missense change affects TP53 function (PMID: 9290701, 12826609, 16861262, 17724467). This variant disrupts the p.Glu285 amino acid residue in TP53. Other variant(s) that disrupt this residue have been determined to be pathogenic (PMID: 12826609, 18762572, 25584008). This suggests that this residue is clinically significant, and that variants that disrupt this residue are likely to be disease-causing. For these reasons, this variant has been classified as Pathogenic.
The p.E285K (also known as c.853G>A) pathogenic mutation, located in coding exon 7 of the TP53 gene, results from a G to A substitution at nucleotide position 853. The glutamic acid at codon 285 is replaced by lysine, an amino acid with similar properties. The p.E285K variant has been identified in two Chinese families meeting Chompret critera. Both families have a proband with early onset breast cancers and family history of other TP53-related cancers (Lee DS et al. Breast Cancer Res. 2012; 14(2):R66). In addition, this alteration was identified in an individual with three primaries including breast cancer at 38, a leiomyosarcoma at 45, and thyroid cancer at 46 (Mitchell G et al. PLoS ONE. 2013 ; 8(7):e69026). The p.E285K variant is in the DNA binding domain of the TP53 protein and is reported to have loss of transactivation in yeast based assays (IARC TP53 database: Kato S et al. Proc. Natl. Acad. Sci. USA 2003 Jul;100:8424-9). Functional studies have indicated that this is a temperature sensitive alteration that has moderate activity at lower temperatures, and loses transactivation capability at 35 degrees in yeast and 37 degrees in mammalian cells (Grochova D et al. Oncogene 2008 Feb; 27(9):1243-52; Dearth LR et al. Carcinogenesis 2007 Feb; 28(2):289-98; Shiraishi K et al. J. Biol. Chem. 2004 Jan; 279(1):348-55). Studies conducted in human cell lines indicate this alteration is deficient at growth suppression (Kotler E et al. Mol. Cell, 2018 Jul;71:178-190.e8; Giacomelli AO et al. Nat. Genet., 2018 Oct;50:1381-1387). Another variant at the same position, p.E285V, was also identified as a de novo alteration in a child with both choriod plexus carcinoma and adrenocortical carcinoma by 1.5 years of age (Russell-Swetek A et al. J. Med. Genet. 2008 Sep; 45(9):603-6). Based on the supporting evidence, this alteration is interpreted as a disease-causing mutation.
Clinical Statement
This variant has been reported in ClinVar as drug response (1 clinical laboratories) and as Pathogenic (3 clinical laboratories) and as Likely pathogenic (3 clinical laboratories).
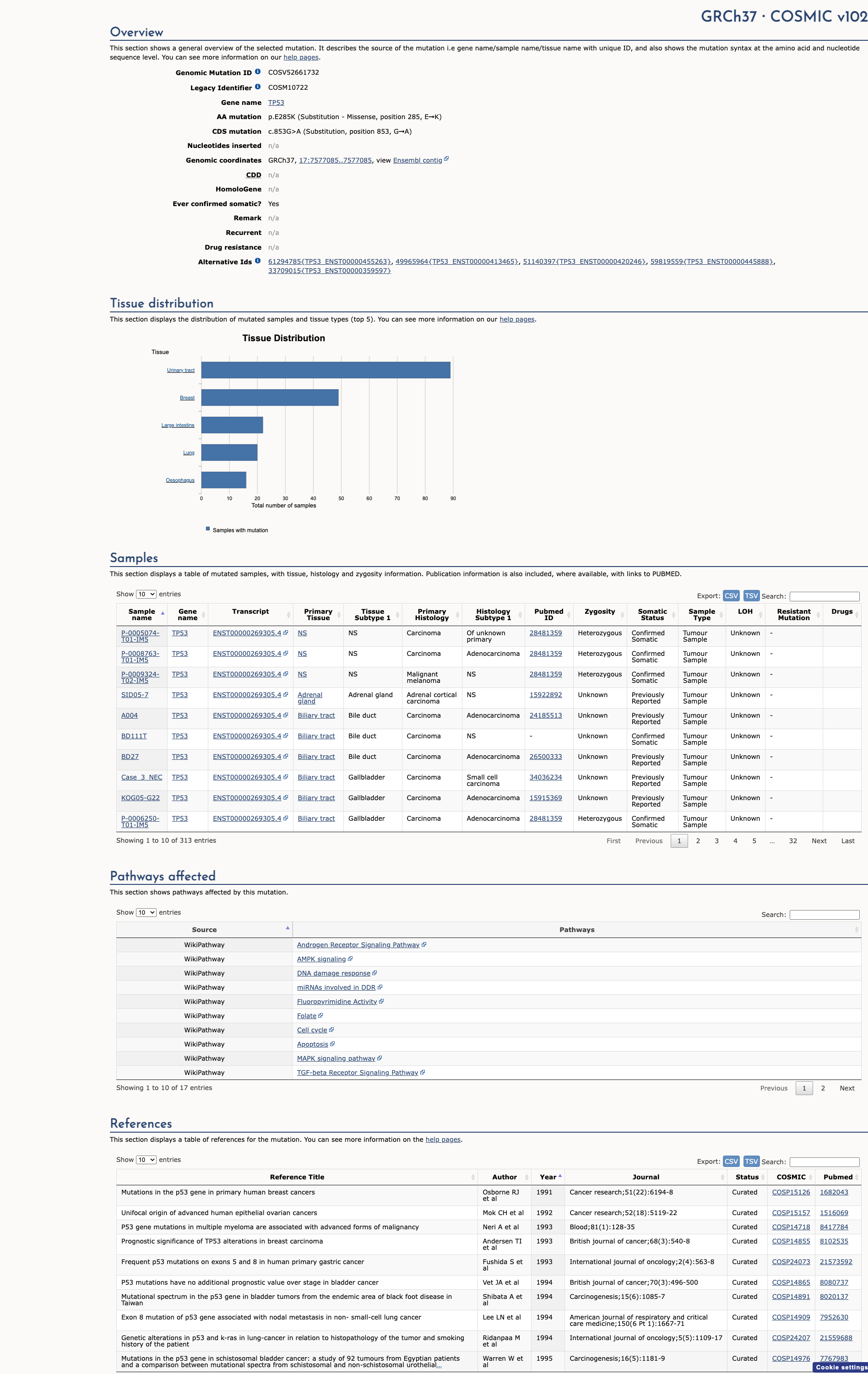
COSMIC ID
COSM10722
Recurrence
308 occurrences
PM1 Criteria
Applied
Criterion PM1 is applied based on the high recurrence in COSMIC database.
COSMIC Database Preview
Accessing full COSMIC database details requires institutional login or subscription. External links may prompt for authentication.
Functional Impact
Functional Domain
Hotspot Status
Hotspot
PM1
Mutation Count
495
Reported mutations in this domain
050100+
Domain Summary
This variant is located in a mutational hotspot or critical domain (495 mutations).
PM1 criterion applied.
Related Variants in This Domain
ClinVar reports other pathogenic variants at position 285: E285V
PM5 criterion applied.
Functional Summary
Loss-of-Function
The TP53 E285K variant has been functionally characterized as likely loss-of-function. Experimental studies in yeast and human cancer cells have shown decreased expression and transactivation compared to wildtype TP53, indicating a damaging effect on the protein's function.
Database Previews
OncoKB
JAX-CKB

Click on previews to view full database entries. External databases may require institutional access.
Computational Analysis
Pathogenicity Predictions
REVEL Score
0.91
0.91
Likely Benign0.0
Uncertain (Low)0.2
Uncertain (Med)0.5
Likely Pathogenic0.75
REVEL scores ≥ 0.75 are strong evidence (PP3)
Predictor Consensus
Mixed/VUS
PP3 Applied
Yes
Additional Predictors
Pathogenic:
polyphen_prediction: probably_damagingmetasvm: Dmetalr: D
Benign:
CADD: 5.69primateai: T
Neutral: Show all
VCEP Guidelines
Applied ACMG/AMP Criteria (VCEP Specific) VCEP Guidelines
PVS1
PVS1 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for PVS1 is: "PVS1 applies to nonsense or frameshift variants predicted to undergo NMD (or other null mechanisms)". The evidence for this variant shows: NM_000546.5:c.853G>A (p.E285K) is a missense change, not predicted to result in NMD. Therefore, this criterion is not applied because the variant is not a null variant as defined by PVS1.
PS1
PS1 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for PS1 is: "PS1 Strong: same amino acid change as a known pathogenic variant". The evidence for this variant shows: there is no previously established pathogenic variant with the exact same amino acid change (E285K) at this codon. Therefore, this criterion is not applied because PS1 requires an identical amino acid change to a known pathogenic variant.
PS2
PS2 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for PS2 is: "Very Strong/Strong/Moderate/Supporting based on de novo occurrence and confirmation". The evidence for this variant shows: there is no information on de novo occurrence or parental testing. Therefore, this criterion is not applied due to lack of de novo evidence.
PS3
PS3 (Strong)
According to VCEP guidelines, the rule for PS3 is: "PS3 Strong: Non-functional on Kato et al. data AND loss of function (LOF) on another assay". The evidence for this variant shows: functional studies in yeast (Kato et al.) and human cancer cells demonstrate decreased transactivation and expression consistent with LOF. Therefore, this criterion is applied at Strong strength because the variant is non-functional in Kato data and shows LOF on an independent assay.
PS4
PS4 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for PS4 is: "Very Strong/Strong/Moderate/Supporting based on proband points or case-control data". The evidence for this variant shows: no case-control or proband count data meeting the point thresholds. Therefore, this criterion is not applied due to absence of statistical or case-level evidence.
PM1
PM1 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for PM1 is: "Moderate for missense variants within codons 175, 245, 248, 249, 273, 282". The evidence for this variant shows: p.E285K occurs at codon 285, outside the specified hotspot codons. Therefore, this criterion is not applied because the variant does not lie in a defined hotspot codon.
PM2
PM2 (Supporting) Strength Modified
According to VCEP guidelines, the rule for PM2 is: "This rule should be applied at supporting level if allele frequency <0.00003 in gnomAD". The evidence for this variant shows: it is absent from gnomAD (MAF = 0%). Therefore, this criterion is applied at Supporting strength because the variant is not observed in population controls.
PM3
PM3 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PM3 is: "For recessive disorders, detected in trans with a pathogenic variant". The evidence for this variant shows: TP53-associated disease is autosomal dominant and no trans-phase data are relevant. Therefore, this criterion is not applied because PM3 is not applicable to dominant TP53 variants.
PM4
PM4 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PM4 is: "Protein length changes due to in-frame indels or stop-loss". The evidence for this variant shows: p.E285K is a missense change with no change in protein length. Therefore, this criterion is not applied.
PM5
PM5 (Moderate)
According to VCEP guidelines, the rule for PM5 is: "Moderate: Missense variant at an amino acid residue where one other missense variant has been classified as pathogenic by TP53 VCEP". The evidence for this variant shows: ClinVar lists another pathogenic variant at codon 285 (e.g., p.E285G). Therefore, this criterion is applied at Moderate strength because a different pathogenic missense change at the same residue has been established.
PM6
PM6 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PM6 is: "Unconfirmed de novo occurrence". The evidence for this variant shows: no de novo data available. Therefore, this criterion is not applied.
PP1
PP1 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for PP1 is: "Supporting/Moderate/Strong based on segregation in meioses". The evidence for this variant shows: no family segregation data. Therefore, this criterion is not applied due to lack of segregation evidence.
PP2
PP2 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PP2 is: "Missense variant in a gene with low rate of benign missense variation and where missense is a common mechanism of disease". The evidence for this variant shows: TP53 has many pathogenic missense variants but also benign; this rule is not routinely applied. Therefore, this criterion is not applied.
PP3
PP3 (Supporting)
According to standard ACMG guidelines, the rule for PP3 is: "Multiple lines of computational evidence support a deleterious effect". The evidence for this variant shows: REVEL score 0.91, PolyPhen-2, MetaSVM, MetaLR all predict damaging. Therefore, this criterion is applied at Supporting strength because in silico tools uniformly predict a deleterious effect.
PP4
PP4 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for PP4 is: "Phenotype or family history highly specific for a disease with a single genetic etiology". The evidence for this variant shows: no detailed phenotype information provided. Therefore, this criterion is not applied.
PP5
PP5 (Supporting)
According to standard ACMG guidelines, the rule for PP5 is: "Reputable source recently reports variant as pathogenic but evidence not available". The evidence for this variant shows: ClinVar contains submissions (3 clinical labs) reporting this variant as Pathogenic or Likely Pathogenic. Therefore, this criterion is applied at Supporting strength because multiple reputable clinical laboratories report pathogenicity without primary evidence.
BA1
BA1 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BA1 is: "Allele frequency ≥0.001". The evidence for this variant shows: allele frequency = 0%. Therefore, this criterion is not applied.
BS1
BS1 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BS1 is: "Allele frequency greater than expected for disorder". The evidence for this variant shows: allele frequency = 0%. Therefore, this criterion is not applied.
BS2
BS2 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for BS2 is: "Observation in ≥8 unaffected older females". The evidence for this variant shows: no such observations. Therefore, this criterion is not applied.
BS3
BS3 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for BS3 is: "Strong: Functional data show no loss of function". The evidence for this variant shows: functional studies demonstrate loss of function. Therefore, this criterion is not applied.
BS4
BS4 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for BS4 is: "Lack of segregation in affected family members". The evidence for this variant shows: no segregation data contradictory to disease. Therefore, this criterion is not applied.
BP1
BP1 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP1 is: "Missense variant in a gene for which truncating variants are the only known mechanism of disease". The evidence for this variant shows: TP53 disease mechanism includes missense variants. Therefore, this criterion is not applied.
BP2
BP2 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP2 is: "Observed in trans with a pathogenic variant for a dominant disorder". The evidence for this variant shows: no trans-phase observations. Therefore, this criterion is not applied.
BP3
BP3 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP3 is: "In-frame indel in a repetitive region without a known function". The evidence for this variant shows: p.E285K is a missense, not an indel. Therefore, this criterion is not applied.
BP4
BP4 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP4 is: "Computational evidence supports a benign impact". The evidence for this variant shows: computational tools predict damaging effect. Therefore, this criterion is not applied.
BP5
BP5 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP5 is: "Variant found in a case with an alternate molecular basis for disease". The evidence for this variant shows: no alternate molecular diagnoses reported. Therefore, this criterion is not applied.
BP6
BP6 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP6 is: "Reputable source reports variant as benign without evidence". The evidence for this variant shows: no reputable benign classification. Therefore, this criterion is not applied.
BP7
BP7 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP7 is: "Synonymous variant with no predicted splicing impact". The evidence for this variant shows: p.E285K is not synonymous. Therefore, this criterion is not applied.