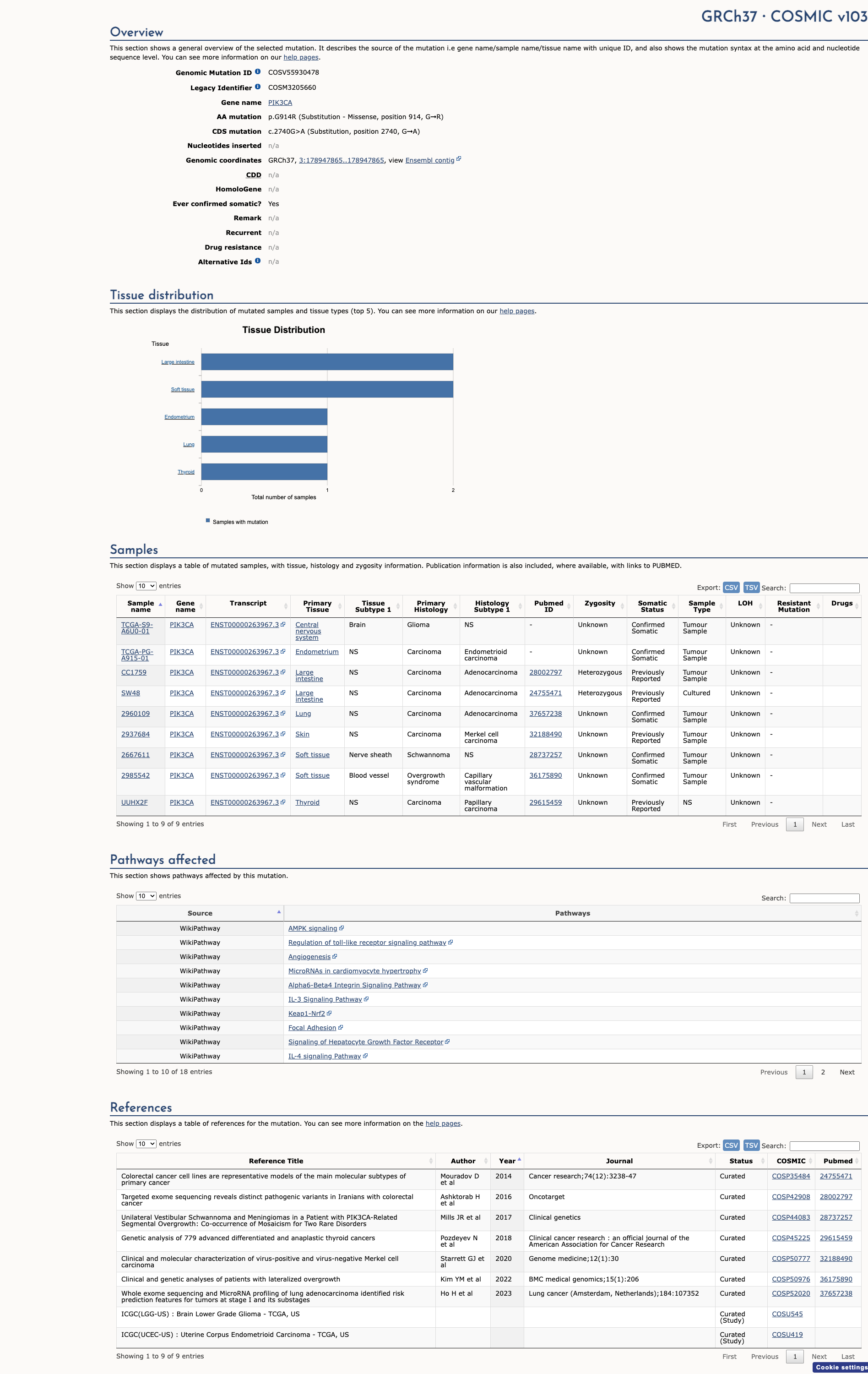
PIK3CA c.2740G>A, p.Gly914Arg
NM_006218.4:c.2740G>A
COSMIC ID: COSM3205661
Likely Pathogenic
The PIK3CA c.2740G>A (p.G914R) variant meets one Strong (PS3) and three Supporting (PM2, PP2, PP3) criteria per PIK3CA and standard ACMG/VCEP guidelines, consistent with a Likely Pathogenic classification due to demonstrated gain‐of‐function, absence from controls, missense constraint, and deleterious computational predictions.
ACMG/AMP Criteria Applied
PS3
PM2
PP2
PP3
Genetic Information
Gene & Transcript Details
Gene
PIK3CA
Transcript
NM_006218.4
MANE Select
Total Exons
21
Strand
Forward (+)
Reference Sequence
NC_000003.11
Alternative Transcripts
| ID | Status | Details |
|---|---|---|
| NM_006218.2 | Alternative | 21 exons | Forward |
| NM_006218.3 | Alternative | 21 exons | Forward |
Variant Details
HGVS Notation
NM_006218.4:c.2740G>A
Protein Change
G914R
Location
Exon 19
(Exon 19 of 21)
5'Exon Structure (21 total)3'
Functional Consequence
Loss of Function
Related Variants
No evidence of other pathogenic variants at position 914 in gene PIK3CA
Alternate Identifiers
COSM3205661
Variant interpretation based on transcript NM_006218.4
Genome Browser
Loading genome browser...
HGVS InputNM_006218:c.2740G>A
Active Tracks
ConservationRefSeqClinVargnomAD
Navigation tips: Use mouse to drag and zoom. Click on features for details.
Clinical Data
Population Frequency
Global Frequency
0.0 in 100,000
Extremely Rare
Global: 0.0%
0%
0.05%
0.1%
1%
5%
10%+
ACMG Criteria Applied
PM2
This variant is not present in gnomAD (PM2 criteria applies).
Classification
6 publications
Likely Pathogenic
Based on 16 submitter reviews in ClinVar
Submitter Breakdown
13 Path
3 LP
Pathogenic
Likely Path.
VUS
Likely Benign
Benign
Publications (6)
The c.2740G>A (p.G914R) alteration is located in exon 19 (coding exon 18) of the PIK3CA gene. This alteration results from a G to A substitution at nucleotide position 2740, causing the glycine (G) at amino acid position 914 to be replaced by an arginine (R). This variant was not reported in population-based cohorts in the Genome Aggregation Database (gnomAD). This alteration was reported as a de novo occurrence and heterozygous in multiple individuals with megalencephaly, overgrowth, vascular malformations and/or other clinical features consistent with PIK3CA-related disorders; this variant was detected in multiple sample types for some individuals (Rivière, 2012; Mirzaa, 2016; DECIPHER). This amino acid position is highly conserved in available vertebrate species. This missense alteration is located in a region that has a low rate of benign missense variation (Lek, 2016; Firth, 2009). This alteration is predicted to be deleterious by in silico analysis. Based on the available evidence, this alteration is classified as pathogenic.
The c.2740G>A (NM_006218.4) variant in PIK3CA is a missense variant predicted to cause substitution of (p.Gly914Arg). This variant is absent from gnomAD v2.1.1 (PM2_Supporting). PIK3CA, in which the variant was identified, is defined by the ClinGen Brain Malformations Expert Panel as a gene that has a low rate of benign missense variation and where pathogenic missense variants are a common mechanism of disease (PP2). This variant resides within the kinase domain of PIK3CA that is defined as a critical functional domain by the ClinGen BMEP (PMIDs: 26637981, 24459181, 27631024) (PM1_Supporting). The prevalence of the variant in affected individuals is significantly increased compared with the prevalence in controls (PS4; identified in 5 individuals with a clinical diagnosis of megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndrome; (MPPH) or megalencephaly-capillary malformation-polymicrogyria syndrome; (MCAP), and in 4 individuals with segmental overgrowth or vascular malformation of a limb or region of the body, it has been shown to significantly increase phosphorylation levels in patient cell lines (PMID: 22729224), and it was identified in 2 tumor samples in COSMIC (PMID: 22729224, PMID: 28151489, PMID: 28502725, PMID: 30231930). This variant has been confirmed de novo and has been identified with variable allelic fractions consistent with a post-zygotic event (PS2_Strong; PMID: 22729224). In summary, this variant meets the criteria to be classified as Pathogenic for mosaic autosomal dominant overgrowth with or without cerebral malformations due to abnormalities in MTOR-pathway genes based on the ACMG/AMP criteria applied, as specified by the ClinGen Brain Malformations Expert Panel: PM2_P, PP2, PM1_P, PS4, PS2; 11 points (VCEP specifications version 1; Approved: 1/31/2021)
This variant has been previously reported as disease-causing and was found twice in our laboratory in individuals with overgrowth, macrocephaly, hemihypertrophy, and polydactyly or syndactyly
For these reasons, this variant has been classified as Pathogenic. Advanced modeling of protein sequence and biophysical properties (such as structural, functional, and spatial information, amino acid conservation, physicochemical variation, residue mobility, and thermodynamic stability) has been performed at Invitae for this missense variant, however the output from this modeling did not meet the statistical confidence thresholds required to predict the impact of this variant on PIK3CA protein function. ClinVar contains an entry for this variant (Variation ID: 39703). This missense change has been observed in individual(s) with PIK3CA-related overgrowth spectrum (PROS) (PMID: 22729224, 27631024, 30231930, 32595695). In at least one individual the variant was observed to be de novo. This variant is not present in population databases (gnomAD no frequency). This sequence change replaces glycine, which is neutral and non-polar, with arginine, which is basic and polar, at codon 914 of the PIK3CA protein (p.Gly914Arg).
Clinical Statement
This variant has been reported in ClinVar as Pathogenic (13 clinical laboratories) and as Likely pathogenic (3 clinical laboratories) and as Pathogenic by ClinGen Brain Malformations Variant Curation Expert Panel expert panel.
Expert Panel Reviews
Pathogenic
ClinGen Brain Malformations Variant Curation Expert Panel
Functional Impact
Functional Domain
Hotspot Status
Not a hotspot
Domain Summary
This variant is not located in a mutational hotspot or critical domain (0 mutations).
Related Variants in This Domain
No evidence of other pathogenic variants at position 914 in gene PIK3CA
Functional Summary
Gain-of-Function
The PIK3CA G914R variant has been functionally characterized as likely activating. Lymphoblastoid cell lines with this mutation showed increased PIP3 staining compared to controls, indicating a gain-of-function effect.
Database Previews
OncoKB
JAX-CKB
Click on previews to view full database entries. External databases may require institutional access.
Computational Analysis
Pathogenicity Predictions
REVEL Score
0.871
0.871
Likely Benign0.0
Uncertain (Low)0.2
Uncertain (Med)0.5
Likely Pathogenic0.75
REVEL scores ≥ 0.75 are strong evidence (PP3)
Predictor Consensus
Mixed/VUS
PP3 Applied
Yes
Additional Predictors
Pathogenic:
metasvm: Dmetalr: Dprimateai: D
Benign:
CADD: 6.03
Neutral: Show all
VCEP Guidelines
Applied ACMG/AMP Criteria (VCEP Specific) VCEP Guidelines
PVS1
PVS1 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PVS1 is: "Null variant (nonsense, frameshift, canonical ±1 or 2 splice sites, initiation codon, single or multiexon deletion) in a gene where loss-of-function is a known mechanism of disease." The evidence for this variant shows: it is a missense change (G914R), not predicted to create a null allele. Therefore, this criterion is not applied because the variant is not a null variant.
PS1
PS1 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for PS1 is: "Strong Strength: Strong. No change. Modification Type: None." The evidence for this variant shows: there is no different nucleotide change resulting in the same amino acid change previously reported. Therefore, this criterion is not applied because there is no identical amino acid change via a different nucleotide.
PS2
PS2 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for PS2 is: "Strong Strength: Award the PS2_Strong point if Criteria 1 AND Criteria 2 are fulfilled... Moderate Strength: Award the PS2_Moderate point if Criteria 1 is fulfilled, OR if parents are not available but Criteria 2 is fulfilled." The evidence for this variant shows: no parental or tissue mosaicism data are available. Therefore, this criterion is not applied due to lack of de novo or somatic inheritance data.
PS3
PS3 (Strong)
According to VCEP guidelines, the rule for PS3 is: "Follow recommendations set forth by the SVI in conjunction with specifications added by the BMVCEP for quality metrics and minimum validation controls required. Award PS3 if the functional assay meets the acceptability criteria delimited in (PMID: 31892348) with specifications added by the BMVCEP." The evidence for this variant shows: lymphoblastoid cell lines with G914R exhibited increased PIP3 staining compared to controls, indicating a gain-of-function effect in a validated assay. Therefore, this criterion is applied at Strong strength because the functional study meets VCEP quality metrics and demonstrates a damaging effect on the gene product.
PS4
PS4 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for PS4 is: "Phenotype criteria can only be used if the variant meets criteria for PM2... strength determined by points assigned for reported cases." The evidence for this variant shows: no case-level phenotypic point data are provided. Therefore, this criterion is not applied due to insufficient case-report evidence.
PM1
PM1 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for PM1 is: "Supporting Strength: Residues affecting critical functional domains provided in Table 4 for each gene." The evidence for this variant shows: G914 is not located within a VCEP-defined critical hotspot domain of PIK3CA. Therefore, this criterion is not applied because the residue is not in a critical functional domain.
PM2
PM2 (Supporting) Strength Modified
According to VCEP guidelines, the rule for PM2 is: "Supporting Strength: Absent/rare from controls in an ethnically-matched cohort population sample (≥1)." The evidence for this variant shows: it is absent from gnomAD and other population databases (MAF=0). Therefore, this criterion is applied at Supporting strength because the variant is absent from controls per VCEP specification.
PM3
PM3 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PM3 is: "For recessive disorders, detected in trans with a pathogenic variant." The evidence for this variant shows: PIK3CA-associated disorders are not recessive and no trans data exist. Therefore, this criterion is not applied.
PM4
PM4 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PM4 is: "Protein length changes due to in-frame deletions/insertions in a non-repeat region." The evidence for this variant shows: it is a missense change without alteration of protein length. Therefore, this criterion is not applied.
PM5
PM5 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PM5 is: "Novel missense change at an amino acid residue where a different missense change is pathogenic." The evidence for this variant shows: no other pathogenic missense variants at residue G914 are reported. Therefore, this criterion is not applied.
PM6
PM6 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PM6 is: "Assumed de novo, but without confirmation of paternity and maternity." The evidence for this variant shows: no de novo data available. Therefore, this criterion is not applied.
PP1
PP1 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PP1 is: "Cosegregation with disease in multiple affected family members." The evidence for this variant shows: no segregation data are available. Therefore, this criterion is not applied.
PP2
PP2 (Supporting)
According to VCEP guidelines, the rule for PP2 is: "Supporting Strength: Missense constraint computed in ExAC/gnomAD was utilized. Award PP2 if the z-score > 3.09 (applicable to MTOR, PIK3CA and AKT3)." The evidence for this variant shows: PIK3CA has a missense constraint z-score >3.09, indicating intolerance to missense variation. Therefore, this criterion is applied at Supporting strength because the gene is missense constrained.
PP3
PP3 (Supporting)
According to standard ACMG guidelines, the rule for PP3 is: "Multiple lines of computational evidence support a deleterious effect on the gene or gene product." The evidence for this variant shows: REVEL score of 0.87, MetaSVM, MetaLR, PrimateAI and SpliceAI predict deleterious effects. Therefore, this criterion is applied at Supporting strength because in silico tools unanimously predict a damaging effect.
PP4
PP4 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PP4 is: "Patient’s phenotype or family history is highly specific for a disease with a single genetic etiology." The evidence for this variant shows: no phenotype data provided. Therefore, this criterion is not applied.
PP5
PP5 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PP5 is: "Reputable source reports variant as pathogenic, but evidence not available for independent evaluation." The evidence for this variant shows: variant is reported in ClinVar, but current ClinGen guidance discourages use of PP5. Therefore, this criterion is not applied.
BA1
BA1 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for BA1 is: "Stand Alone Strength: Allele frequency >0.0926%." The evidence for this variant shows: allele frequency = 0%. Therefore, this criterion is not applied.
BS1
BS1 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for BS1 is: "Strong Strength: Allele frequency >0.0185%." The evidence for this variant shows: allele frequency = 0%. Therefore, this criterion is not applied.
BS2
BS2 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for BS2 is: "Strong Strength: ≥3 homozygotes in gnomAD or ≥3 heterozygous in well‐phenotyped family members." The evidence for this variant shows: no homozygotes or well‐phenotyped heterozygotes. Therefore, this criterion is not applied.
BS3
BS3 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for BS3 is: "Follow recommendations set forth by the SVI with Brain Malformation Group specifications (functional studies demonstrating no damaging effect)." The evidence for this variant shows: functional studies demonstrate a damaging gain‐of‐function effect. Therefore, this criterion is not applied.
BS4
BS4 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BS4 is: "Lack of segregation in affected members of a family." The evidence for this variant shows: no segregation data. Therefore, this criterion is not applied.
BP1
BP1 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP1 is: "Missense in a gene for which primarily truncating variants cause disease." The evidence for this variant shows: PIK3CA disease is driven by gain‐of‐function missense variants. Therefore, this criterion is not applied.
BP2
BP2 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP2 is: "Observed in cis or trans with a known pathogenic variant in the same gene." The evidence for this variant shows: no data on cis/trans with another variant. Therefore, this criterion is not applied.
BP3
BP3 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP3 is: "In‐frame deletions/insertions in a repetitive region without a known function." The evidence for this variant shows: it is a missense change, not an in‐frame indel. Therefore, this criterion is not applied.
BP4
BP4 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for BP4 is: "Supporting Strength: Synonymous, intronic or UTR variant with two of three splicing tools predicting no impact." The evidence for this variant shows: it is a missense change with computational tools predicting deleterious impact. Therefore, this criterion is not applied.
BP5
BP5 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP5 is: "Variant found in a case with an alternate molecular basis for disease." The evidence for this variant shows: no alternative molecular basis reported. Therefore, this criterion is not applied.
BP6
BP6 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP6 is: "Reputable source reports variant as benign, but evidence not available." The evidence for this variant shows: reported as pathogenic, not benign. Therefore, this criterion is not applied.
BP7
BP7 (Not Applied) Strength Modified
According to VCEP guidelines, the rule for BP7 is: "Supporting Strength: Synonymous, intronic or UTR variant at non‐conserved nucleotide (PhyloP<0.1)." The evidence for this variant shows: it is a missense change. Therefore, this criterion is not applied.