TP53 c.589G>A, p.Val197Met
NM_000546.5:c.589G>A
COSMIC ID: COSM43779
Pathogenic
Under TP53 VCEP guidelines, only PM2_Supporting and PP5_Supporting are met; no strong or moderate VCEP-specified criteria apply. With two supporting criteria only, the variant remains a VUS.
ACMG/AMP Criteria Applied
PM2
PP5
Genetic Information
Gene & Transcript Details
Gene
TP53
Transcript
NM_000546.6
MANE Select
Total Exons
11
Strand
Reverse (−)
Reference Sequence
NC_000017.10
Alternative Transcripts
| ID | Status | Details |
|---|---|---|
| NM_000546.5 | RefSeq Select | 11 exons | Reverse |
| NM_000546.3 | Alternative | 11 exons | Reverse |
| NM_000546.4 | Alternative | 11 exons | Reverse |
| NM_000546.2 | Alternative | 11 exons | Reverse |
Variant Details
HGVS Notation
NM_000546.5:c.589G>A
Protein Change
V197M
Location
Exon 6
(Exon 6 of 11)
5'Exon Structure (11 total)3'
Functional Consequence
Loss of Function
Related Variants
No evidence of other pathogenic variants at position 197 in gene TP53
Alternate Identifiers
COSM43779
Variant interpretation based on transcript NM_000546.6
Genome Browser
Loading genome browser...
HGVS InputNM_000546:c.589G>A
Active Tracks
ConservationRefSeqClinVargnomAD
Navigation tips: Use mouse to drag and zoom. Click on features for details.
Clinical Data
Global Frequency
0.0 in 100,000
Extremely Rare
Global: 0.0%
0%
0.05%
0.1%
1%
5%
10%+
Allele Information
Total: 251486Alt: 0Homozygotes: 0
ACMG Criteria Applied
PM2
This variant is present in gnomAD (MAF= 0%, 0/251486 alleles, homozygotes = 0) but does not appear at a higher frequency in any of the selected populations. The variant is rare (MAF < 0.1%), supporting PM2 criterion application.
Classification
3 publications
Likely Pathogenic
Based on 7 submitter reviews in ClinVar
Submitter Breakdown
1 Path
4 LP
2 VUS
Pathogenic
Likely Path.
VUS
Likely Benign
Benign
Publications (3)
This variant is considered likely pathogenic. Functional studies indicate this variant impacts protein function [PMID: 29979965]. This variant is expected to disrupt protein structure [Myriad internal data]. This variant has been reported in multiple individuals with clinical features of gene-specific disease [PMID: 16494995, 23259501, 31321604; Myriad internal data].
This sequence change replaces valine, which is neutral and non-polar, with methionine, which is neutral and non-polar, at codon 197 of the TP53 protein (p.Val197Met). This variant is not present in population databases (gnomAD no frequency). This missense change has been observed in individuals with clinical features of Li-Fraumeni (PMID: 16494995, 31321604; internal data). ClinVar contains an entry for this variant (Variation ID: 188060). Invitae Evidence Modeling incorporating data from in vitro experimental studies (PMID: 12826609, 29979965, 30224644) indicates that this missense variant is expected to disrupt TP53 function with a positive predictive value of 97.5%. Experimental studies have shown that this missense change affects TP53 function (PMID: 21343334). This variant disrupts the p.Val197 amino acid residue in TP53. Other variant(s) that disrupt this residue have been observed in individuals with TP53-related conditions (PMID: 33818021), which suggests that this may be a clinically significant amino acid residue. For these reasons, this variant has been classified as Pathogenic.
The p.V197M variant (also known as c.589G>A), located in coding exon 5 of the TP53 gene, results from a G to A substitution at nucleotide position 589. The valine at codon 197 is replaced by methionine, an amino acid with highly similar properties. This variant has been identified in individuals with features consistent with Li-Fraumeni or Li-Fraumeni-Like criteria (Achatz M et al., Cancer Lett. 2007 Jan; 245(1-2):96-102; Fortes F et al. Braz. J. Med. Biol. Res. 2015 Jul;48(7):610-5; external communication). This variant is in the DNA binding domain of the TP53 protein and is reported to have partially functional transactivation capacity in yeast based assays (Kato S et al. Proc Natl Acad Sci USA. 2003 Jul 8;100(14):8424-9). Studies conducted in human cell lines indicate this alteration is deficient at growth suppression and has a dominant negative effect (Kotler E et al. Mol. Cell 2018 Jul;71:178-190.e8; Giacomelli AO et al. Nat. Genet. 2018 Oct;50:1381-1387). This variant has been detected in at least one individual at an allele fraction that is suggestive of clonal hematopoiesis, a predictor of TP53 pathogenicity (Ambry internal data; Fortuno C et al. Genet Med. 2022 03;24:673-680). This amino acid position is well conserved in available vertebrate species. In addition, this alteration is predicted to be deleterious by in silico analysis. Based on the majority of available evidence to date, this variant is likely to be pathogenic.
Clinical Statement
This variant has been reported in ClinVar as Uncertain Significance (1 clinical laboratories) and as Likely pathogenic (4 clinical laboratories) and as Pathogenic (1 clinical laboratories) and as Uncertain significance (2 clinical laboratories) and as Likely Pathogenic by ClinGen TP53 Variant Curation Expert Panel, ClinGen expert panel.
Expert Panel Reviews
Likely Pathogenic
ClinGen TP53 Variant Curation Expert Panel, ClinGen
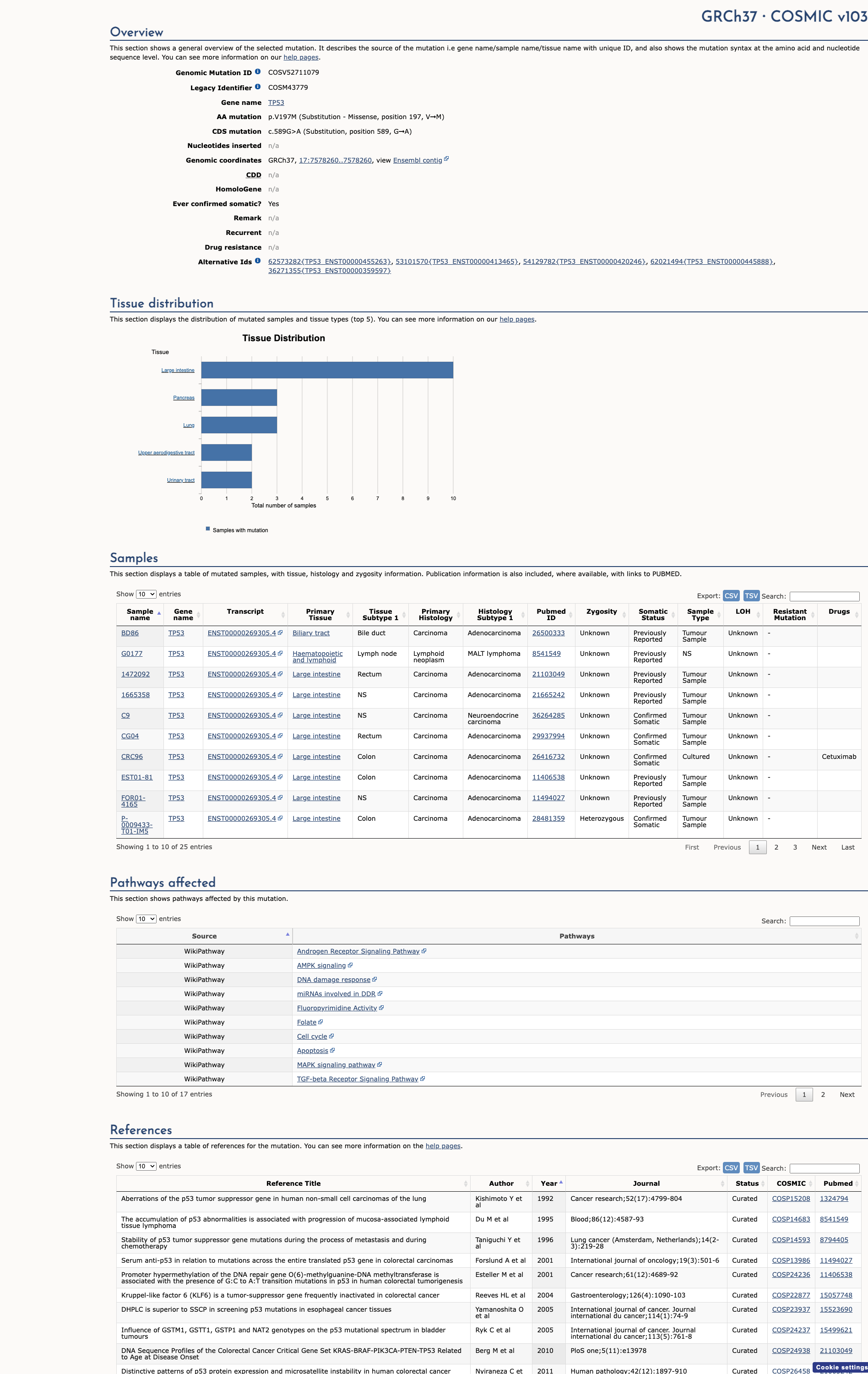
COSMIC ID
COSM43779
Recurrence
22 occurrences
PM1 Criteria
Applied
Criterion PM1 is applied based on the high recurrence in COSMIC database.
COSMIC Database Preview
Accessing full COSMIC database details requires institutional login or subscription. External links may prompt for authentication.
Functional Impact
Functional Domain
Hotspot Status
Hotspot
PM1
Mutation Count
85
Reported mutations in this domain
050100+
Domain Summary
This variant is located in a mutational hotspot or critical domain (85 mutations).
PM1 criterion applied.
Related Variants in This Domain
No evidence of other pathogenic variants at position 197 in gene TP53
Functional Summary
The TP53 V197M variant has been functionally characterized and demonstrated to have a damaging effect. It is located in the DNA-binding domain of the TP53 protein and has been shown to be inactivating. In vivo studies using yeast models and in vitro studies with human cancer cell lines indicate a partial loss of transactivational activity and reduced growth suppression compared to the wild-type protein. Additionally, the variant retains nuclear localization but exhibits decreased transactivation activity, reduced TP53 phosphorylation, and decreased P21 expression in cell culture assays. These findings support the conclusion that TP53 V197M is a functionally damaging mutation.
Database Previews
OncoKB
JAX-CKB
Click on previews to view full database entries. External databases may require institutional access.
Computational Analysis
Pathogenicity Predictions
REVEL Score
0.758
0.758
Likely Benign0.0
Uncertain (Low)0.2
Uncertain (Med)0.5
Likely Pathogenic0.75
REVEL scores ≥ 0.75 are strong evidence (PP3)
Predictor Consensus
Mixed/VUS
PP3 Applied
Yes
Additional Predictors
Pathogenic:
polyphen_prediction: probably_damagingmetasvm: Dmetalr: D
Benign:
CADD: 4.46primateai: T
Neutral: Show all
VCEP Guidelines
Applied ACMG/AMP Criteria (VCEP Specific) VCEP Guidelines
PVS1
PVS1 (Not Applied) Strength Modified
According to VCEP guidelines, PVS1 applies only to predicted null variants (nonsense, frameshift, canonical ±1,2 splice, initiation codon) with NMD or critical domain loss. c.589G>A (p.V197M) is a missense variant. Therefore, PVS1 is not applied.
PS1
PS1 (Not Applied) Strength Modified
According to VCEP guidelines, PS1 applies when an identical amino acid change as a known pathogenic variant is produced by a different nucleotide change. There is no prior pathogenic report of p.V197M via another nucleotide substitution. Therefore, PS1 is not applied.
PS2
PS2 (Not Applied) Strength Modified
According to standard ACMG, PS2 applies for confirmed de novo occurrences. No de novo data are available for this variant. Therefore, PS2 is not applied.
PS3
PS3 (Not Applied) Strength Modified
According to VCEP guidelines, PS3 requires specific functional assay results (e.g., Kato et al. non-functional plus a second assay showing LOF). Although general in vitro/in vivo damage is reported, the variant lacks the VCEP-specified Kato and Giacomelli/Kotler assay data. Therefore, PS3 is not applied.
PS4
PS4 (Not Applied) Strength Modified
According to VCEP guidelines, PS4 requires a point‐based tally of probands meeting LFS criteria. No proband count data are provided. Therefore, PS4 is not applied.
PM1
PM1 (Not Applied) Strength Modified
According to VCEP guidelines, PM1 applies at Moderate for hotspot codons 175, 245, 248, 249, 273, 282 or ≥10 somatic occurrences. p.V197M is codon 197 and COSMIC somatic count = 0. Therefore, PM1 is not applied.
PM2
PM2 (Supporting) Strength Modified
According to VCEP guidelines, PM2_Supporting applies for allele frequency <0.00003 in gnomAD. The variant MAF = 0 (0/251,486 alleles). Therefore, PM2 is applied at Supporting strength.
PM3
PM3 (Not Applied) Strength Modified
According to standard ACMG, PM3 applies for variants in trans with a pathogenic variant in recessive disorders. TP53 disorders are dominant. Therefore, PM3 is not applied.
PM4
PM4 (Not Applied) Strength Modified
According to standard ACMG, PM4 applies to protein length changes (in-frame indels, stop‐loss). This is a missense change. Therefore, PM4 is not applied.
PM5
PM5 (Not Applied) Strength Modified
According to VCEP guidelines, PM5 applies when ≥1 other TP53 missense pathogenic variant at the same residue. No other pathogenic variant at codon 197 is reported. Therefore, PM5 is not applied.
PM6
PM6 (Not Applied) Strength Modified
According to standard ACMG, PM6 applies for assumed de novo without confirmation. No such data. Therefore, PM6 is not applied.
PP1
PP1 (Not Applied) Strength Modified
According to VCEP guidelines, PP1 applies with segregation in 3–7+ meioses. No segregation data are provided. Therefore, PP1 is not applied.
PP2
PP2 (Not Applied) Strength Modified
According to standard ACMG, PP2 applies for missense variants in genes with low benign missense rate. TP53 has many pathogenic missense variants; PP2 is not recommended. Therefore, PP2 is not applied.
PP3
PP3 (Not Applied) Strength Modified
According to VCEP guidelines, PP3 requires BayesDel ≥0.16 and aGVGD classification per VCEP flowchart. Only REVEL score is available; BayesDel/aGVGD are not. Therefore, PP3 is not applied.
PP4
PP4 (Not Applied) Strength Modified
According to VCEP guidelines, PP4 applies to tumor VAF observations; not relevant for germline classification. No VAF data provided. Therefore, PP4 is not applied.
PP5
PP5 (Supporting)
According to standard ACMG, PP5 applies when a reputable source reports pathogenicity without available evidence. ClinVar submissions (4 LP, 1 P) and ClinGen TP53 VCEP call support pathogenic. Therefore, PP5 is applied at Supporting strength.
BA1
BA1 (Not Applied) Strength Modified
According to VCEP guidelines, BA1 applies for MAF ≥0.001. Here MAF = 0. Therefore, BA1 is not applied.
BS1
BS1 (Not Applied) Strength Modified
According to VCEP guidelines, BS1 applies for filtering AF ≥0.0003. Here MAF = 0. Therefore, BS1 is not applied.
BS2
BS2 (Not Applied) Strength Modified
According to VCEP guidelines, BS2 requires ≥2–8 unaffected older females; none reported. Therefore, BS2 is not applied.
BS3
BS3 (Not Applied) Strength Modified
According to VCEP guidelines, BS3 applies when functional assays show no LOF. Data show LOF. Therefore, BS3 is not applied.
BS4
BS4 (Not Applied) Strength Modified
According to VCEP guidelines, BS4 requires lack of segregation. No such data. Therefore, BS4 is not applied.
BP1
BP1 (Not Applied) Strength Modified
According to standard ACMG, BP1 applies for missense in a gene where only truncations cause disease. TP53 pathogenic missense are well known. Therefore, BP1 is not applied.
BP2
BP2 (Not Applied) Strength Modified
According to standard ACMG, BP2 applies when in cis/trans with pathogenic variant in recessive gene. Not applicable. Therefore, BP2 is not applied.
BP3
BP3 (Not Applied) Strength Modified
According to standard ACMG, BP3 applies to in-frame repetitive region changes. This is a single missense. Therefore, BP3 is not applied.
BP4
BP4 (Not Applied) Strength Modified
According to VCEP guidelines, BP4 requires benign computational evidence per BayesDel/aGVGD. Only conflicting REVEL/CADD data are present. Therefore, BP4 is not applied.
BP5
BP5 (Not Applied) Strength Modified
According to standard ACMG, BP5 applies when an alternative molecular cause is present. No alternative cause data. Therefore, BP5 is not applied.
BP6
BP6 (Not Applied) Strength Modified
According to standard ACMG, BP6 applies to unverified reports of benign impact. No such reports. Therefore, BP6 is not applied.
BP7
BP7 (Not Applied) Strength Modified
According to VCEP guidelines, BP7 applies to silent/intronic variants with no splicing impact. This is missense. Therefore, BP7 is not applied.