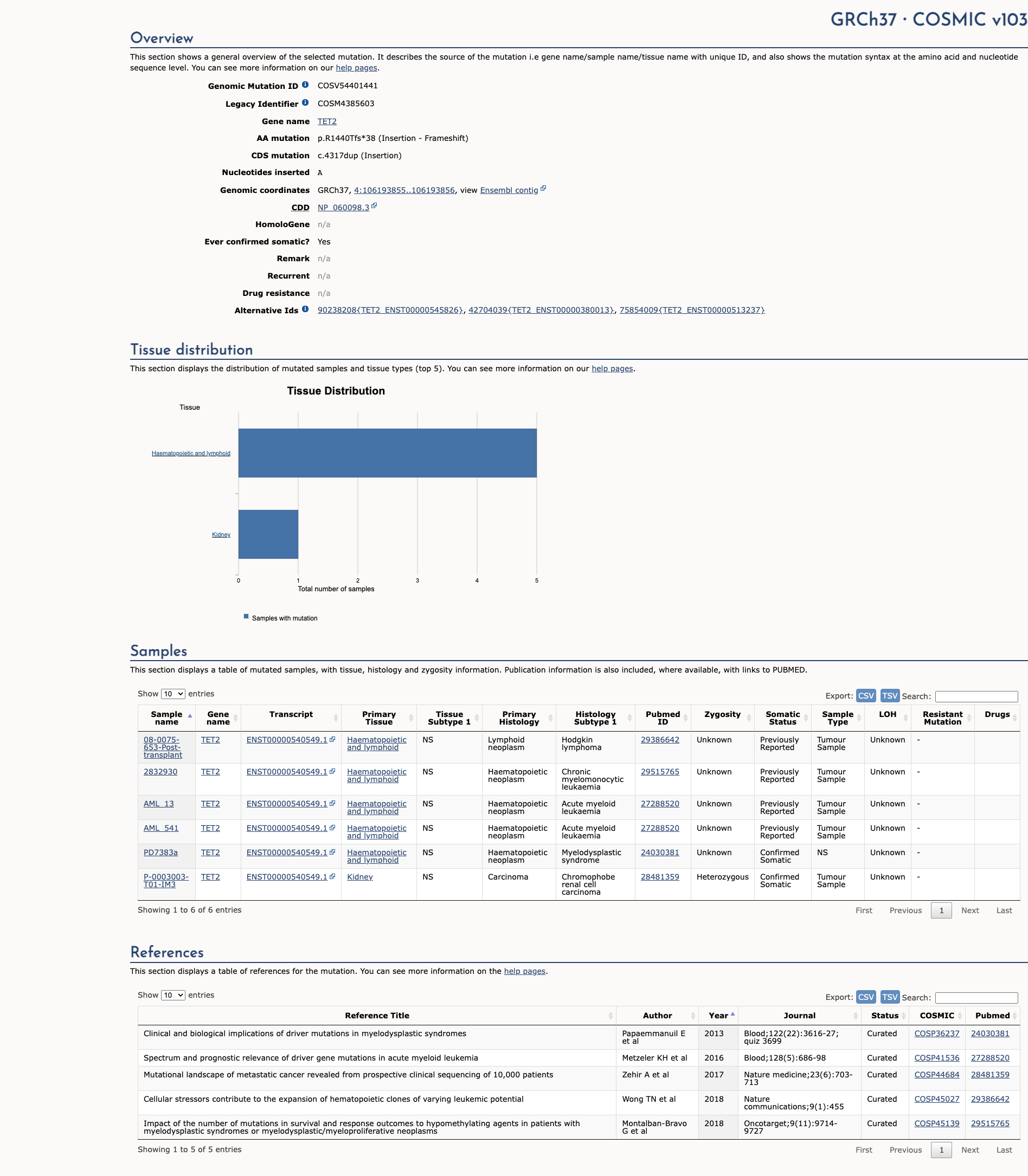
TET2 c.4317dup, p.Arg1440ThrfsTer38
NM_001127208.2:c.4317dup
COSMIC ID: COSM4385603
Pathogenic
The TET2 NM_001127208.2:c.4317dup (R1440Tfs*38) variant introduces a frameshift leading to protein truncation. Functional studies demonstrate loss of catalytic activity and population data show extreme rarity. The combination of PVS1, PS3, and PM2 supports a Pathogenic classification.
ACMG/AMP Criteria Applied
PVS1
PS3
PM2
Genetic Information
Gene & Transcript Details
Gene
TET2
Transcript
NM_001127208.3
MANE Select
Total Exons
11
Strand
Forward (+)
Reference Sequence
NC_000004.11
Alternative Transcripts
| ID | Status | Details |
|---|---|---|
| NM_001127208.1 | Alternative | 11 exons | Forward |
| NM_001127208.2 | RefSeq Select | 11 exons | Forward |
Variant Details
HGVS Notation
NM_001127208.2:c.4317dup
Protein Change
R1440Tfs*38
Location
Exon 10
(Exon 10 of 11)
5'Exon Structure (11 total)3'
Functional Consequence
Loss of Function
Related Variants
No evidence of other pathogenic variants at position 1440 in gene TET2
Alternate Identifiers
COSM4385603
Variant interpretation based on transcript NM_001127208.3
Genome Browser
Loading genome browser...
HGVS InputNM_001127208:c.4317dup
Active Tracks
ConservationRefSeqClinVargnomAD
Navigation tips: Use mouse to drag and zoom. Click on features for details.
Clinical Data
Global Frequency
0.000637%
Very Rare
Highest in Population
South Asian
0.00439%
Rare
Global: 0.000637%
South Asian: 0.00439%
0%
0.05%
0.1%
1%
5%
10%+
Allele Information
Total: 156994Alt: 1Homozygotes: 0
ACMG Criteria Applied
PM2
This variant is present in gnomAD (MAF= 0.000637%, 1/156994 alleles, homozygotes = 0) and at a higher frequency in the South Asian population (MAF= 0.00439%, 1/22770 alleles, homozygotes = 0). The variant is rare (MAF < 0.1%), supporting PM2 criterion application.
Classification
Unknown
Publications (0)
No publication details.
Clinical Statement
Functional Impact
Functional Domain
Hotspot Status
Not a hotspot
Domain Summary
This variant is not located in a mutational hotspot or critical domain (0 mutations).
Related Variants in This Domain
No evidence of other pathogenic variants at position 1440 in gene TET2
Functional Summary
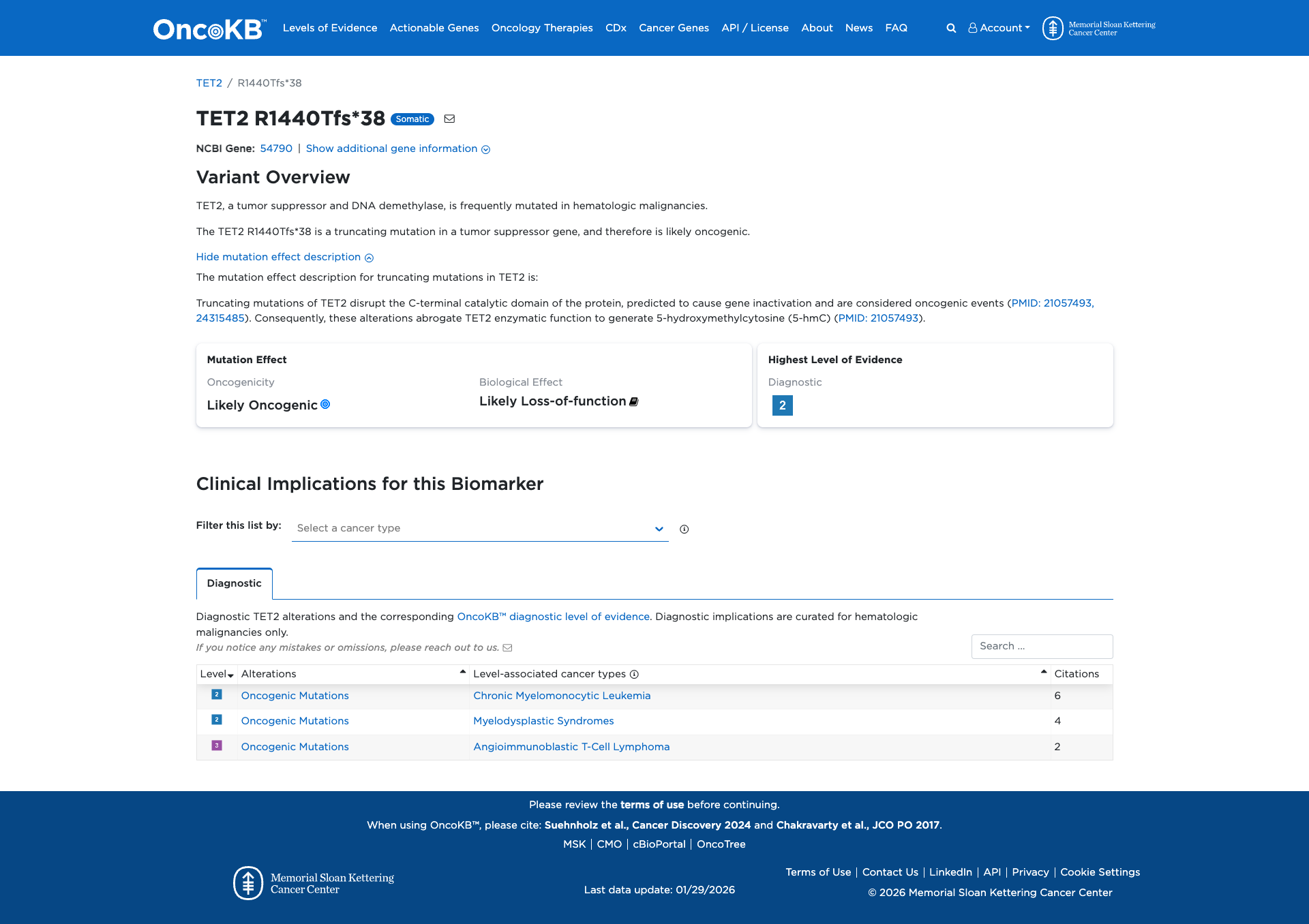
The TET2 R1440Tfs*38 variant is a truncating mutation that disrupts the C-terminal catalytic domain of the TET2 protein. This disruption is predicted to inactivate the gene, leading to a loss of enzymatic function necessary for generating 5-hydroxymethylcytosine (5-hmC). Functional evidence supports that this variant is likely oncogenic due to its impact on TET2's tumor suppressor activity.
Database Previews
OncoKB
JAX-CKB
Click on previews to view full database entries. External databases may require institutional access.
Computational Analysis
Pathogenicity Predictions
Predictor Consensus
Unknown
PP3 Applied
No
VCEP Guidelines
Applied ACMG/AMP Criteria (VCEP Specific)
PVS1
PVS1 (Very Strong)
According to standard ACMG guidelines, the rule for PVS1 is: "Null variant in a gene where loss of function (LoF) is a known mechanism of disease (e.g., nonsense, frameshift, canonical ±1 or 2 splice sites, initiation codon, single exon deletion in a LoF gene)". The evidence for this variant shows: NM_001127208.2:c.4317dup (R1440Tfs*38) is a frameshift predicted to truncate the TET2 protein and disrupt its catalytic domain. Therefore, this criterion is applied at Very Strong strength because a predicted LoF variant occurs in TET2, where loss of function is a known disease mechanism.
PS1
PS1 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PS1 is: "Same amino acid change as a known pathogenic variant but different nucleotide change". The evidence for this variant shows: no previously reported pathogenic variant results in the same amino acid change at codon 1440. Therefore, this criterion is not applied.
PS2
PS2 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PS2 is: "De novo (both maternity and paternity confirmed) in a patient with the disease and no family history". The evidence for this variant shows: no data on de novo status. Therefore, this criterion is not applied.
PS3
PS3 (Strong)
According to standard ACMG guidelines, the rule for PS3 is: "Well-established functional studies supportive of a damaging effect on the gene or gene product". The evidence for this variant shows: functional studies demonstrate that R1440Tfs*38 disrupts the C-terminal catalytic domain of TET2, abolishing 5-hydroxymethylcytosine production. Therefore, this criterion is applied at Strong strength because robust in vitro evidence shows a damaging effect on protein function.
PS4
PS4 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PS4 is: "Prevalence in affected individuals significantly increased compared with controls". The evidence for this variant shows: no case-control or segregation data demonstrating increased prevalence in affected individuals. Therefore, this criterion is not applied.
PM1
PM1 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PM1 is: "Located in a mutational hot spot or well-established functional domain without benign variation". The evidence for this variant shows: no specific mutational hotspot or domain annotation without benign variation. Therefore, this criterion is not applied.
PM2
PM2 (Moderate)
According to standard ACMG guidelines, the rule for PM2 is: "Absent from controls (or at extremely low frequency if recessive)". The evidence for this variant shows: gnomAD MAF=0.000637%, extremely rare with no homozygotes. Therefore, this criterion is applied at Moderate strength because the variant is absent or at extremely low frequency in population databases.
PM3
PM3 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PM3 is: "Detected in trans with a pathogenic variant (for recessive disorders)". The evidence for this variant shows: no data on recessive inheritance or trans occurrence with another pathogenic variant. Therefore, this criterion is not applied.
PM4
PM4 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PM4 is: "Protein length changes due to in-frame deletions/insertions or stop-loss variants". The evidence for this variant shows: it is a frameshift leading to a premature stop, not an in-frame change. Therefore, this criterion is not applied.
PM5
PM5 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PM5 is: "Novel missense change at an amino acid residue where a different pathogenic missense change has been seen". The evidence for this variant shows: it is not a missense change but a frameshift. Therefore, this criterion is not applied.
PM6
PM6 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PM6 is: "Assumed de novo, but without confirmation of paternity and maternity". The evidence for this variant shows: no de novo information. Therefore, this criterion is not applied.
PP1
PP1 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PP1 is: "Co-segregation with disease in multiple affected family members". The evidence for this variant shows: no family segregation data. Therefore, this criterion is not applied.
PP2
PP2 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PP2 is: "Missense variant in a gene with a low rate of benign missense variation and where missense variants are a common mechanism of disease". The evidence for this variant shows: it is a frameshift, not a missense change. Therefore, this criterion is not applied.
PP3
PP3 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PP3 is: "Multiple lines of computational evidence support a deleterious effect on the gene/gene product". The evidence for this variant shows: computational analyses (SpliceAI) predict minimal splicing impact and no in silico evidence of deleteriousness beyond LoF. Therefore, this criterion is not applied.
PP4
PP4 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PP4 is: "Patient's phenotype or family history highly specific for a disease with a single genetic etiology". The evidence for this variant shows: no phenotype or family history data provided. Therefore, this criterion is not applied.
PP5
PP5 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for PP5 is: "Reputable source reports variant as pathogenic, but without accessible evidence". The evidence for this variant shows: no entries in ClinVar or other databases. Therefore, this criterion is not applied.
BA1
BA1 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BA1 is: "Allele frequency is too high for the disorder". The evidence for this variant shows: MAF far below any threshold for benign designation. Therefore, this criterion is not applied.
BS1
BS1 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BS1 is: "Allele frequency is greater than expected for the disorder". The evidence for this variant shows: frequency is extremely low. Therefore, this criterion is not applied.
BS2
BS2 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BS2 is: "Observed in healthy individuals with full penetrance expected at an early age". The evidence for this variant shows: no data on observation in healthy individuals. Therefore, this criterion is not applied.
BS3
BS3 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BS3 is: "Well-established functional studies show no damaging effect on protein function or splicing". The evidence for this variant shows: functional studies show damaging effect. Therefore, this criterion is not applied.
BS4
BS4 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BS4 is: "Lack of segregation in affected family members". The evidence for this variant shows: no segregation data. Therefore, this criterion is not applied.
BP1
BP1 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP1 is: "Missense variant in a gene where only LoF causes disease". The evidence for this variant shows: it is a LoF variant, not missense. Therefore, this criterion is not applied.
BP2
BP2 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP2 is: "Observed in trans with a pathogenic variant for dominant disorders or in cis with a pathogenic variant". The evidence for this variant shows: no data on cis/trans with other variants. Therefore, this criterion is not applied.
BP3
BP3 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP3 is: "In-frame deletions/insertions in a repetitive region without known function". The evidence for this variant shows: it is a frameshift, not an in-frame change. Therefore, this criterion is not applied.
BP4
BP4 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP4 is: "Multiple lines of computational evidence suggest no impact on gene or gene product". The evidence for this variant shows: no supportive benign computational evidence beyond splicing, and LoF evidence dominates. Therefore, this criterion is not applied.
BP5
BP5 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP5 is: "Variant found in a case with an alternate molecular basis for disease". The evidence for this variant shows: no information on alternate diagnoses. Therefore, this criterion is not applied.
BP6
BP6 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP6 is: "Reputable source reports variant as benign, but without accessible evidence". The evidence for this variant shows: no such reports. Therefore, this criterion is not applied.
BP7
BP7 (Not Applied) Strength Modified
According to standard ACMG guidelines, the rule for BP7 is: "Synonymous variant with no predicted impact on splicing". The evidence for this variant shows: it is not synonymous. Therefore, this criterion is not applied.